What is the Hatch-Waxman Act?
The Drug Price Competition and Patent Term Restoration Act, or the Hatch-Waxman Act, is a legal framework authorized by the Congress in 1984.
The Act came into force to streamline the approval process of generic drugs and preserve innovation incentives and establish a procedure for litigation involving generic pharmaceuticals. The Hatch-Waxman Act established a legal as well as the economic foundation for the US generic drug industry.
The Act offers drug developers some protection while facilitating and giving incentives for companies to file an Abbreviated New Drug Application (ANDA).
Hatch-Waxman balance out between innovation and affordability. The Act also offers incentives to drug manufacturing companies for R&D of innovative medicines. Moreover, Hatch-Waxman offers affordability by developing an abbreviated path for generic drug developers to bring pharmaceuticals to market at a lower cost.
In the US, many top-selling prescription medicines are currently the subject of patent challenges by some generic firms seeking to enter the market under the Hatch-Waxman Act, some of them are-
- Singulair - Treatment of asthma or allergy.
- Lovenox - To treat deep vein thrombosis / pulmonary embolism.
- Abilify - Schizophrenia, bipolar disorder, and depressive disorder drug.
What are the basics of the Hatch-Waxman Act?
The Hatch-Waxman Act regulates the approval procedure, along with the marketing of generic drugs. Under this Act, the marketing authorization procedure for generic drugs is streamlined.
The Act permits generic drug developers to file an ANDA incorporating the safety and effectiveness data submitted by the brand drug manufacturer. Only bioequivalence studies need to be added. Moreover, generic manufacturers can develop and evaluate the product without any worries of an infringement action by the patent holder.
The Hatch-Waxman Act provisions are exclusively applicable to pharmaceutical patents and are distinct from infringement procedures related to the other patented products and processes.
New drug manufacturers were given protections in two ways:
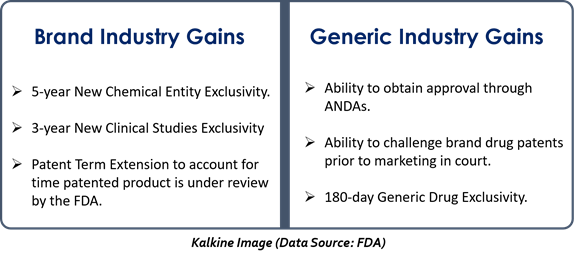
- By providing market exclusivity to the drug innovator- With a five-year data exclusivity to a new chemical entity, a new kind of market exclusivity was introduced. During the exclusivity period, the Food and Drug Administration (FDA) cannot authorize a generic version of an innovative drug.
- Extension in the life of patents- The Act allows an extension in patents life, covering the time a drug is under FDA regulatory review. This makes sure that regulatory review will not consume the life of a patent.
Key components of the Hatch-Waxman Act
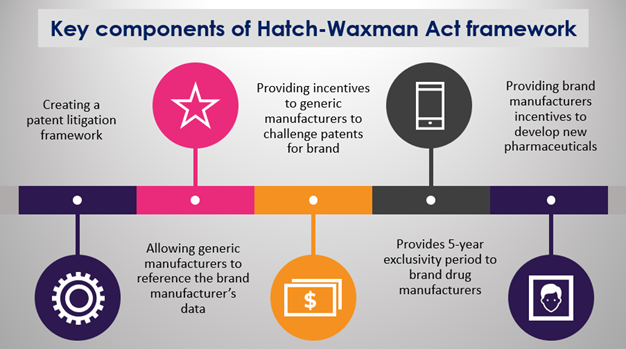
Source: Copyright © 2021 Kalkine Media Pty Ltd.
How does the Hatch-Waxman Act work?
Hatch-Waxman Act requires the pharmaceutical company to declare how the activities will relate to Orange Book-listed patents on drug’s marketing, before filing its ANDA.
According to the Act, a company submitting a new drug application (NDA) needs to provide the patent information of that drug in the Orange Book. This document offers generic drug developers an accessible list of approved drugs for which an ANDA could be filed.
Besides, a generic firm must certify its intentions to the FDA about each patent on the Orange Book for the generic drug it seeks to market.
There are four possibilities under the Hatch-Waxman Act:
- The patent on the drug was never filed.
- Patents listed in the Orange Book have expired.
- The company will not market the drug until all the listed patents have expired.
- The patents listed are invalid or irrelevant.
What are the benefits of the Act?
The Hatch-Waxman Act has promoted innovation, fostered competition, and facilitated the US remains a leader in healthcare sector research and development.
Since Hatch-Waxman’s enactment, the US generic drug industry has witnessed enormous growth. Moreover, biopharmaceutical players have continued to develop innovative treatments for complex indications.
With the Hatch-Waxman Act’s help, the US is the frontrunner in medical innovation, while balancing affordability and innovation, and promoting competition.
Before the Hatch-Waxman Act, the time for a generic drug to enter the market was three to five years, after the expiry of patents for the brand name drugs. However, with the Act’s enactment, generic drugs often enter the market immediately on patent expiration.
Moreover, the Act includes incentives for the manufacturers of both generic as well as branded drugs.
 Please wait processing your request...
Please wait processing your request...